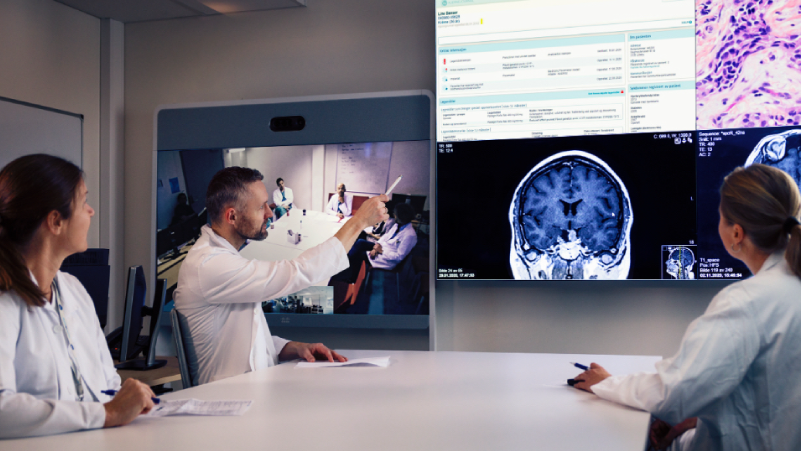
Beacon of the Cancer Care – PCM4EU – Personalised Cancer Medicine for all EU citizens
ombining expertise across borders to promote Precision Cancer Medicine in Europe.
The PCM4EU is a project under Europe’s Beating Cancer Plan by EU4Health, with partners from 15 countries across Europe. PCM4EU is about facilitating implementation of molecular cancer diagnostics for precision oncology, and the consortium consists of partners from altogether 15 countries across Europe.

Millions of people in the EU are negatively affected by cancer, and cancer is one of the leading causes of death and morbidity in Europe. Personalised Cancer Medicine (PCM) is an emerging approach where patients are given more precise and targeted diagnostics and treatment. However, the approach depends on access to adequate molecular diagnostics and drugs to have impact and move towards implementation. Today there is still inequality in access to PCM between and within EU countries, and while the promise of PCM is clear, implementation remains a challenge.
With 3.7 million new cases each year, cancer is one of the leading causes of death and morbidity in the EU. Personalised cancer medicine (PCM) has the potential to beat this disease. But currently, there is inequality in access to PCM between and within EU Member States.

As PCM is based on targeting driver mutations in tumours, its success depends on access to:
- next-generation sequencing-based molecular diagnostics,
- clinical decision support systems,
- trials to facilitate implementation of the PCM approach.
PCM4EU aims to improve the survival rates and quality of life of cancer patients in the EU using PCM based on best practices. The project consortium has a good track record in the implementation of molecular diagnostics in academically-led cross-border PCM trials in north-western Europe. This expertise will be made available to support a wider use and access to PCM in Europe.

PCM4EU will:
- evaluate current standards and provide best practice guidelines and recommendations on implementation and interpretation of state-of-the-art genomic diagnostics, supporting their implementation and interpretation;
- support development of harmonised European mechanisms for interpretation of molecular and clinical data so that information from cohorts of patients with rare mutations/tumour types in several countries can be analysed simultaneously to obtain faster answers;
- ensure cross-border access to PCM;
- facilitate the incorporation of the project results into healthcare systems in a cost-effective way for future patients;
- provide education for all stakeholders, including physicians, pathologists, patients and decision makers.

![]()
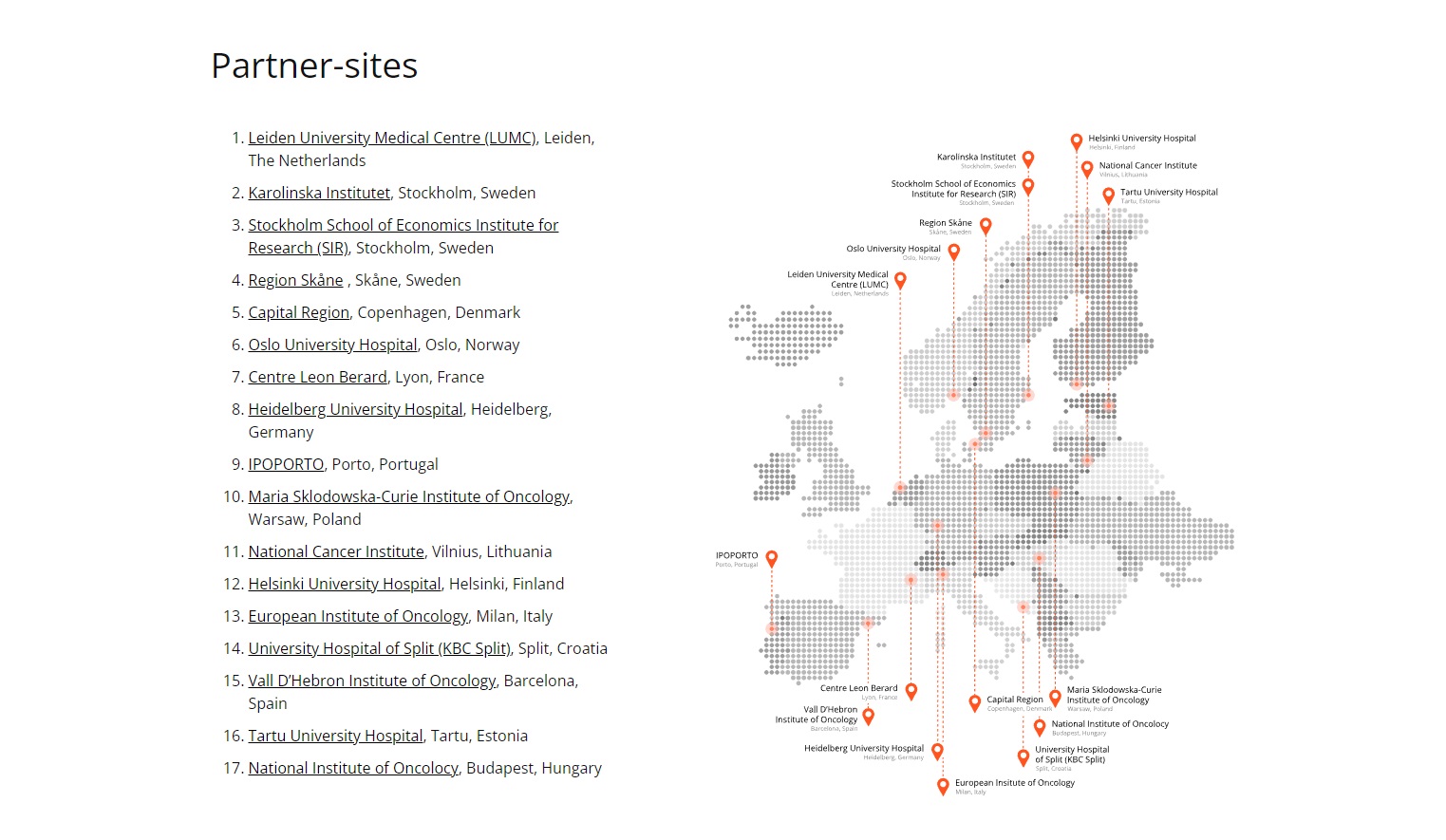
Partners of “Beacon of the Cancer Care”